Australia Reach Regulation for Various Sectors
The main obligation is on the Australian Manufacturers and importers.
Registration of Industrial chemical introducers: Under AICIS, introducers of industrial chemicals shall register their businesses before the introduction of industrial chemicals. Introduction includes both manufacture and import of chemicals in Australia. Introducers must register with the Register of Industrial Chemical Introducers for a registration year before introducing an industrial chemical during that year. Name and address of introducer will appear in the Register. Per year registration charges are application for this registration. If the introducer introduces a chemical without registration then penalties apply.
Australia replced the existing chemical regulation NICNAS to a new scheme Australian Industrial Chemicals Introduction Scheme - AICIS from 1st July 2020.
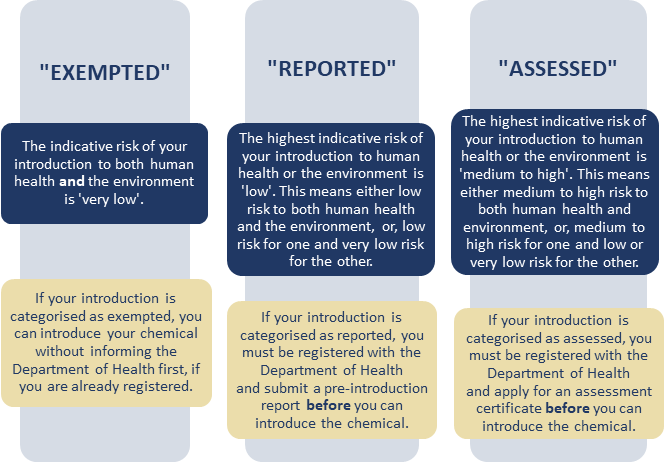
AICIS regulates chemicals with an industrial use and requires introducers of industrial chemicals to register their businesses before introduction. Introductions include both manufacture and import.
This regulation is implemented for the protection of human health and the environment through restriction, regulation, assessment, evaluation and by managing risks arising due to introduction hence ensures the safe use of the industrial chemical. Purpose of AICIS is to maintain a Register of Industrial Chemical Introducers.
Scope
This Scheme only registers industrial chemicals which used in inks, plastics, adhesives, paints, glues, solvents, cosmetics, soaps and many other products. This act does not apply to chemicals which are used in agricultural and veterinary or therapeutic purposes, or in food or feed. It only promotes safe use of industrial chemicals by providing information and recommendations to other regulators.
A manufacturer or importer (introducer) of industrial chemicals or products that release industrial chemicals — into Australia for commercial purposes must register their business if not alread registered with previous regulation NICNAS. This business registration shall be complete within given registration year before import or manufacture (introduce) of an industrial chemical. Registration year is 1 September to 31 August.
List of registered businesses will be published on the website of Australian Governement and will include the name and address of introducers.
Penalties apply if a person introduces an industrial chemical without being registered.
It is important for Non-Australian introducers to register their business and meet compliance & reporting requirements for uninterrupted exports to Australia. Australian Registered Business Number (ARBN) is mandatory for the same. Non-Australian introducers can also appoint an Australian agent for compliance requirements if they are unable to obtain Australian Registered Business Number (ARBN).
Anyone who is registered under AICIS to introduce industrial chemicals or products containing industrial chemicals (for example, cosmetics, paints and plastics) must submit an annual declaration. This applies to all of your chemical introductions, regardless of the category, amount or number of chemicals.
Applicants can submit their annual declaration from 1 August. The due date to submit it is 30 November after the end of each registration year.
The Australian Pesticides and Veterinary Medicines Authority is responsible for the registration, approval, and restriction of agrochemicals sold in Australia (APVMA). The use of pesticides is regulated by the Environmental Protection Agency (EPA) under the Pesticides Act of 1999 and Regulation 2017. The EPA, Commonwealth and NSW government agencies, as well as other stakeholder organisations, oversee pesticide management to guarantee no harm to human health or the environment.
Authorties
APVMA administers Australia’s agvet legislation and has a duty to not only manage potential risks, but also enforce compliance when required.
The legislation includes:
-
the Agricultural and Veterinary Chemicals Act 1994 (the Agvet Act)
-
Agricultural and Veterinary Chemicals Code scheduled to the Agricultural and Veterinary Chemicals Code Act 1994 (the Agvet Code)
-
the Agricultural and Veterinary Chemicals (Administration) Act 1992 (the Admin Act)
-
the Agricultural and Veterinary Chemical Products (Collection of Levy) Act 1994 (the Collection Act).
APVMA has the following duties:
-
issue substantiation notices
-
issue formal warnings
-
enter enforceable undertakings
-
issue enforceable directions
-
issue infringement notices
-
issue stop supply and recall notices
-
cancel or suspend permits for noncompliance or prior convictions
-
cancel or suspend permits to prevent imminent risk
-
issue a notice to attend, give information, or produce documents or things
-
obtain and execute monitoring warrants
-
obtain and execute investigation warrants.
Companies involved in the manufacturing, formulation, repackaging, exportation, importation, marketing, and application service of pesticides in Australia shall file the registration application
Agricultural and Veterinary Chemicals (Administration) Act 1992
The APVMA is established as an independent Commonwealth statutory authority responsible for the regulation and management of agricultural and veterinary chemicals in Australia up to the point of retail sale under this Act. This Act provides all of the internal details of the APVMA's establishment, as well as its functions and powers. It also includes laws governing chemical import and export, as well as enforcement and inspections.
Agricultural and Veterinary Chemicals Act 1994
This act provides the constitutional and other legal elements necessary for the Agvet Code's implementation. The Agvet Code is to be applied as a law of the participating areas, according to the agreement. The Australian Capital Territory and any other territory designated as a participating territory by Regulations in force under section 25 of the act are considered participating territories.
Agricultural and Veterinary Chemicals Code Act 1994
The Agvet Code is included as a schedule in this Act. The Agvet Code provides extensive rules that enable the APVMA to analyze, authorize, register, and review active ingredients and agricultural and veterinary chemical products (and their accompanying labels), as well as give permits and licenses for chemical product manufacturing. It also includes regulations to manage the supply of chemical products, as well as provisions to ensure that the Agvet Code is followed and enforced.
The National Registration Authority for Agricultural and Veterinary Chemicals (NRA) later became the APVMA. The AGVET Code is used by the APVMA of the participating states and territories for addressing the registration process of the active ingredients or chemicals.
In July 1991, the Commonwealth, states and territories agreed for establishing the National Registration Scheme (NRS) for agricultural and veterinary chemicals. The development of the NRS contributes to the process of assessment and registration of all AGVET chemical products which was independently undertaken by the commonwealth states and territories.
The APVMA partners between the Commonwealth and the states and territories within which the establishment of NRA and APVMA took place as a Commonwealth statutory authority, that is responsible for the evaluation, registration and review of agricultural and veterinary chemicals and their control.
The APVMA is also termed as an independent statutory authority that implements the legislative powers, functions and AGVET Codes.
The product label is the primary and most effective means of communicating to users' critical information about pesticide and veterinary drug use that is both safe and effective. Current pesticide and veterinary medicine labelling, on the other hand, is complex, confusing, and inflexible, with separate labelling codes and information for different statutory needs. Because labels have become more extensive and comprehensive, vital information can be difficult to locate. Furthermore, updating information on labels takes time, which can result in different labels for the same product along the supply chain.
By focusing pre-market regulatory effort on those label features that are unique to pesticides and veterinary pharmaceuticals, the Panel advises simplifying labelling and expediting label evaluation. Content that does not require assessment would be subject to the label.
The Australian Pesticides and Veterinary Medicines Authority (APVMA) is in charge of regulating agricultural and veterinary (agvet) chemicals (active components) and the goods that contain them in Australia, up to and including retail sales.
The Agvet Code specifies which items and chemicals require registration or approval and which are exempt. The National Registration Scheme for Agricultural and Veterinary Chemicals in Australia is the regulatory framework for controlling agricultural and veterinary (AgVet) chemicals (NRS). The NRS is a collaborative effort between the Commonwealth and the states and territories, with duties shared equally. The NRS is administered by the Australian Pesticides and Veterinary Medicines Authority (APVMA) in collaboration with state and territory government agencies, as well as other Commonwealth agencies, law enforcement, and the judiciary.
APVMA defines a nanomaterial as “A nanomaterial should be an intentionally produced, manufactured or engineered substance with unique properties that are directly caused by size features with 10 per cent or more of the number size distribution of these features lying in the range approximately 1–100 nm (the nanoscale). There should be recognition that biological and EHS issues may require a different size range above 100 nm.”
Both Nano pesticides and veterinary nanomedicines must be compatible with the proposed APVMA working definition of a nanomaterial. There is currently no universal agreement on what constitutes a nano pesticide or a veterinary nanomedicine. Several researchers have raised a variety of concerns that should be considered while defining a definition for nanopesticides15. For example, the nanomaterial in a nano pesticide could be the active ingredient or a non-active 'carrier; the size of a nano pesticide could be larger than the traditionally accepted upper limit of the nanoscale dimension (100 nm); and the durability of a nano pesticide could be either transient or persistent. According to the SCENIHR (2010) study, "further guidance (requirements) unique for the planned rule" may be required. The APVMA working definition of a nanomaterial is intended to have a nanoscale dimension of 100 nm as the maximum limit; nevertheless, a size range greater than 100 nm may be required to address human health and environmental safety concerns. On a case-by-case basis, the need will be evaluated. For the purposes of risk assessment and APVMA enforcement, the definition applies to Nano pesticides and veterinary nanomedicines.
- Agricultural and Veterinary Chemicals (Administration) Act 1992- The Australian Pesticides and Veterinary Medicines Authority (APVMA) is established as an independent Commonwealth statutory authority responsible for the regulation and management of agricultural and veterinary chemicals in Australia up to the point of retail sale under this Act. This Act provides all the internal details of the APVMA's establishment, as well as its functions and powers. It also includes laws governing chemical import and export, as well as enforcement and inspections.
- Agricultural and Veterinary Chemicals Act 1994- This Act provides the constitutional and other legal elements necessary for the Agvet Code's implementation. The Agvet Code is to be applied as a law of the participating areas, according to the agreement. The Australian Capital Territory and any other territory designated as a participating territory by Regulations in force under section 25 of the Act are considered participating territories.
- Agricultural and Veterinary Chemicals Code Act 1994- The Agvet Code is included as a schedule in this Act. The Agvet Code provides extensive rules that enable the APVMA to analyze, authorize, register, and review active ingredients and agricultural and veterinary chemical products (and their accompanying labels), as well as give permits and licenses for chemical product manufacturing. It also includes regulations to manage the supply of chemical products, as well as provisions to ensure that the Agvet Code is followed and enforced.
Individuals, companies, organisations, trade associations, and other groups can apply online to the APVMA to:
- approve a new active constituent – or change one that has already been approved
- register a new agvet chemical product containing an active constituent – or change one that has already been registered
- approve relevant details of a product label, or change details that have already been approved
- renew a product's registration or the approval on an active constituent
- and obtain a permit.
- obtain an export certificate from the APVMA for an agvet chemical product
- receive pre-application help from the APVMA before applying
- have an APVMA technical assessment completed before applying
- have an APVMA decision reviewed
Before filing an application, have the APVMA conduct a technical examination. Have the APVMA's decision reviewed.
Within a month of receiving an application, the APVMA conducts a preliminary assessment. This is to see if the application fits the application requirements, in other words, if the applicant has provided the APVMA with all the necessary information and paperwork. If this is not the case, additional information may be asked.
The APVMA then evaluates each application within the timeframe set by law before deciding whether to approve, register, or deny it.
The APVMA takes a scientific, evidence-based approach to regulation, aligning its efforts with the hazards posed by each active element or product. Both the likelihood of exposure and the potential effects of exposure are considered when assessing risks.
Approval and register
The APVMA allows databases and publishes certain information regarding products and active constituents on our PubCRIS. Some of this data is made public throughout the evaluation process, while others are made public after approval or registration.
The pertinent details of a product's label, such as the product name and key ingredients – as well as the safety and usage guidelines – are some of the information presented on each database record. If it has been included as a reference product in another application, the information released will indicate which products it has been listed for.
The APVMA is responsible for maintaining the PubCRIS and permits databases, as well as updating records as needed to reflect variations or modifications to a product, permit, licence, label, or active constituent.
The person, corporation, or organisation in charge of a registered chemical product – or an approved active constituent – is accountable for adhering to the Agvet Legislation on an ongoing basis. This can include ensuring that their product or active constituent's labelling, advertising, manufacturing, importing, sales, and supply are all compliant with Agvet regulations.
The APVMA has specific authorities to oversee and monitor compliance with Agvet regulations, as well as to carry out enforcement actions as necessary. Law enforcement, the court, and other state, territory, and Australian government departments work together to enforce the law.
This can involve ensuring that agvet chemical products and active ingredients are sold, supplied, imported, exported, manufactured, labelled, packaged, stored, and advertised in accordance with the law. The APVMA is also in charge of performing chemical reviews, monitoring reports of Agvet regulation non-compliance, and examining allegations of adverse reactions to agvet chemical goods.
APVMA has set the maximum limit for chemical review up to 57 months. The time frame depends on factors to be reviewed such as efficacy, safety, chemistry, etc.
The APVMA has yet to issue any formal rules on the registration and regulation of nanomaterials-containing products. When the person applies to register a product that contains nanomaterials, the basic guideline is that one should include information that addresses the same criteria that APVMA evaluates when registering conventional products. Additional information about the nanomaterial, as well as any hazards that are different from or in addition to the primary hazard, should be included. APVMA will be able to deploy risk management measures on a case-by-case basis as a result of these.
“Until detailed guidelines are available, any person contemplating registering an agvet chemical product containing nanomaterials should first contact the APVMA for guidance on data specific to that product. Any advice given will reflect the practices current at the time for this developing science.”
Persistent Organic Pollutants (POPs), also referred to as “emerging contaminants”, include a range of chemical substances from diverse applications, ranging from medicines, personal care, household cleaning products, lawn care, agricultural products, among others.
The toxicity of POPs has been long investigated, and researchers found that these chemicals may have long-range mobility and long-lasting duration, leading to environmental contamination and pollution. POPs are often classified as Substance of Very High Concern (SVHC) with hormone disruption consequences, bioaccumulation and biomagnification through the food chain. The presence of POPs in the environment and in human health can lead to several complications, from mild effects to more serious consequences, for example, reproductive disruptions and immunological disorders.
Since POPs are not easily broken down, a characteristic that guarantees its continuation in the environment, the contaminant consequences are still being investigated. In other words, the emerging contaminants are mainly not from new pollutants that recently entered the environment. Instead, there is a good understanding of contaminants, but the consequences to their exposure are still being discovered.
Countries have created a set of rules and regulations to regulate, control, and mitigate POPs use and its effects on the environment and on human health. The essence of these legislations is to eliminate the production, trading, and use of such chemicals, including storage and waste management.
International effort in addressing POPs starts in late 1990s. The initial protocol to address POPs was implemented in 1998, signing 16 chemicals to phase out according to previously established risk criteria. The Aarhus Protocol also impose parties to reduce their emissions below 1990 levels.
The Stockholm Convention is an international conference specifically for POPs proposed in May 2001 in Sweden (complementing the Aarhus Protocol), entering into force in 2004. Among the objectives, the Stockholm Convention aims to:
- Prohibit, eliminate, and restrict several POPs in the production, use, import, and export.
- Ensure that waste contained POPs are managed safely and in an environmentally sound manner.
- Promote tools for information exchange, public access, awareness and education, research, development and monitoring, reporting, and implementation of plans to fight POPs pollution.
The Stockholm Convention is considered the main international instrument to address POPs. Most countries adhere to the Stockholm Convention.
Australia adopted the Stockholm Convention in 2004, imposing on 12 original POPs trading control on import, manufacture, use, and export throughout the territory. The Department of Agriculture, Water and the Environment (DAWE) supervises the application of the convention in such chemicals. Also, the Department ensures that POPs added to the convention on later amendments are recognized in Australia. However, Australia does not automatically adopt new amendment to the Stockholm Convention. It is necessary that the internal administration proposes the modifications though a legal text. Currently, the list of POPs ratified by the Australian government are:
- Aldrin
- Chlordane
- Dieldrin
- Endrin
- Heptachlor
- Hexachlorobenzene (HCB)
- Mirex
- Toxaphene
- Polychlorinated biphenyls (PCB)
- DDT
- Dioxins
- Furans
Further, POPs provisions depend on the chemical category, i.e., if it is a pesticide or industrial chemical. It is also to be noted that chemicals may present additional compliance/control measures depending on the local state and territory government in which the chemical is intended to be present on the market.
In addition, the Australia government is creating several efforts to enforce and comply with the Stockholm Convention’s mandate. This includes several guidelines and national plans for chemicals, which highlights:
- The Guidelines for Disposal of waste containing POPs
- The Organochlorine Pesticide Management Plan
- The National Action Plan for Dioxins
- The Polychlorinated Biphenyls Management Plan
- The Hexachlorobenzene Waste Management Plan
- The Industrial Chemicals Environmental Management Standard
In the near future, all chemicals will be submitted to the administration of the Industrial Chemicals Environmental Management Standard.